The Sanderson Group Webpages
Department of Chemistry
Durham University, Durham, UK
Crystal Structure of a β-Ketoacyl-Acyl Carrier Protein Synthase
| Display Options | |
|
Acyl Transfer
Active Site |
Malonate Decarboxylation |
Olsen, J. G., Kadziola, A., von Wettstein-Knowles, P., Siggaard-Andersen, M. and Larsen, S., Structure, 2001, 9, 233-243.
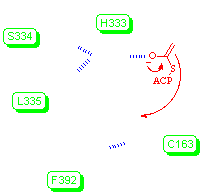
The structure above is a mutant form of the enzyme β-ketoacyl-acyl carrier protein synthase from E. coli, in which Cys163 has been replaced by Serine. As a consequence, the acyl group is still capable of binding to the enzyme as an ester (rather than a thioester), but acyl transfer does not occur (note: you may not see the ester linkage in this example due to an error in the structure file, but it is there). The acyl group binding site consists of a hydrophobic pocket (not shown), and the region around the carbonyl group, in which hydrogen bonding contacts are made to the backbone -NH of Phe392 and the backbone -NH of Ser163. In the unmodified enzyme, the acylated thiol group of Cys163 would be the normal precursor for transfer of the acyl group. The enzyme crystallises as a dimer, with two dimers per unit cell. Only one subunit is shown here to keep file size down.
 |
 |
|
|
|
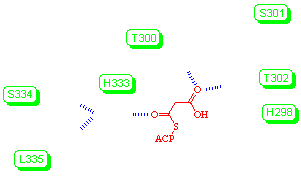
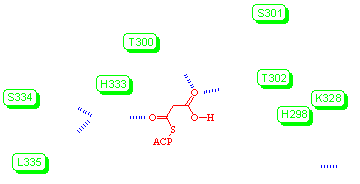
A mechanism (above) is proposed for decarboxylation of malonyl-ACP, in which malonyl-ACP binds to the enzyme through the formation of hydrogen bonds between the thioacyl group and Nε of His333, and between the carboxyl oxo group and the alcoholic groups of Thr300 and Thr302. The other nitrogen of His333, Nδ, accepts hydrogen bonds from the backbone amides of Ser334 and Leu335. His298 is in close contact with the acidic proton of the malonyl group and a hydroxide ion. The hydroxide ion is stabilised by the positive charge of nearby Lys328 and hydrogen bonding to an ordered water molecule. The close distance between the two oxygen nuclei is used as supporting evidence for the presence of the hydroxide ion. The interaction between the hydroxide ion and Nδ of His298 can also be considered as a hydrogen bond. During malonyl-ACP decarboxylation, this hydroxide acts to neutralise the positive charge that accumulates on Nδ of His298. The negatively-charged oxygen of the enolate formed following decarboxylation is stabilised by hydrogen-bonding to Nε of His333.
Acyl transfer involves attack of the enolate formed by decarboxylation of malonyl-ACP on the acyl group of the Cys163 thioester. The key residues in the whole process are the Cys163-His298-His333 catalytic triad. Both of the histidines are involved in the decarboxylation process (above), and Nδ of His333 lies within 3.2 Å of Oγ of Ser163 (or Sγ of Cys163 in the native enzyme.) Attack of the enolate on the acyl group at Cys163 is facilitated by hydrogen bonding of the intermediate tetrahedral anion to the backbone amide of Phe392.